Reading about cellular ageing (Mitochondria) can sound a bit doom and gloom. It seems we’re destined to age by means of an inbuilt “ageing programme” that runs throughout our lives a bit like a piece of software that predisposes us to developing various diseases of ageing as we accumulate birthdays and our cells become “tired”. There’s a lot of talk about inflamm-ageing, a concept that blames inflammation for the consequences of the ageing process. It’s easy to look at inflamm-ageing as unavoidable and thus be tempted to “treat it” almost palliatively. I’m sure you’ve heard about “anti-inflammatory diets”, food supplements and even drugs that are supposed to act as miracle cures for all sort of things and that promise eternal youth just by reducing inflammation. The truth is that by tackling inflammation in this way is superficial and only just about scratches the surface. If you don’t get to the root of the problem, you’ll never be able to find a solution. So tackling cellular ageing from the anti-inflammatory angle can be seen as “cosmetic”.
What is inflammation and where does it come from?
Inflammation is a natural response to stimuli such as pathogenic (bad) bacteria and viruses, as well as to tissue injury or trauma. The latter is most often microscopic in nature and needn’t be noticeable, which is why we’re always exposed to a certain amount of inflammation without necessarily “feeling it” as though we could see the injury with the naked eye.
Inflammation involves a complex process that works in a cascading fashion. Some initial (called “early phase”) proteins (e.g. C-reactive protein and interleukin 6) are released as soon as injury or exposure to infection takes place and these proteins then carry messages that make the expression of further proteins possible.
Without inflammation we wouldn’t be able to deal with the ongoing low grade “attack” that these sources pose to our immune system. As shown in the diagram below, we would either be in a permanent “sickness syndrome” kind of state, or we would go to develop non-resolving inflammation, which normally translates into major diseases, all with an immune component. Let’s leave those for another post…
Is inflammation always bad?
Contrary to popular belief, a little inflammation is not only normal but healthy! Pro-inflamatory cytokines aren’t just random molecules that make things inflamed, and they’re definitely not the bad guys in the movie. They are protein-based messengers with partly immune, partly hormone, partly neurotransmitter-like properties that are needed as part of the communication system that links the brain, with the endocrine and immune systems. Messages from these cytokines travel to the hypothalamus via the bloodstream and the vagus nerve, where they’re processed. The hypothalamus is the “control centre” of the body, a bit like an airport’s air traffic control tower. The messages carried by pro-inflammatory cytokines from the site of infection or injury are processed there, and further instructions are issued to the immune system to either keep up the “red alert status” or whether to chill out for a bit. Without these valuable messages the body wouldn’t be able to reach the dynamic balance we need to experience peak performance and maximum health.
Is this the only source of inflammation?
Well, no. There’s also another major source, the mitochondria, the tiny essential cellular powerhouses that create adenosine triphosphate (ATP), also known as the “cellular energy currency”. Mitochondria use oxidation (technically called “oxidative phosphorylation”, akin to bleaching) in order to “conjure up” energy from nutrients, generating free radicals as by-products of their work.
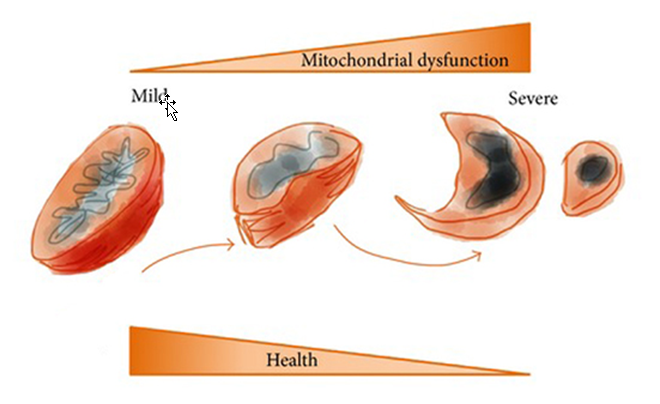
Figure 3. Mitochondrial health and (dys)function. Reproduced with permission from Aging (Albany NY). 2010 Aug;2(8):487-503.
In a world of driven by increasing energy intake (and psychosocial stress… more about this in a moment…) the sheer fact of eating and food metabolism encompasses an unavoidable linkage to oxidation and inflammation by means of free radical damage. Again, some practitioners are obsessed with “dampening down” oxidation and with “fighting free radicals”, which can turn into a bit of a futile battle as oxygen itself becomes a free radical when we breathe it.
The oxygen example illustrates perfectly that free radicals are necessary molecules. In fact, most recently they’ve been identified as key messengers participating in euro-endocrine-immune communication, particularly in the brain itself, where free radicals generated by glial cells participate in the regulation of neuroinflammation. Because free radicals are everywhere, inside and outside us, therapeutic approaches that are exclusively based on “free radical dampening” are essentially flawed. It is even questionable whether silencing the neuro-endocrine-immune messages that these free radicals carry is a good thing in the long run. An interesting question that current neuroendocrinologists and neuroimmunologists are trying to answer and one that I’ll tackle in another post.
Cellular “weather stations”
So the poor little mitochondria get blamed for their continuous churning of free radicals, yet it is in their oxidative nature to do so and it would be deleterious for our health for their function to be “tamed”. Mitochondria are highly dynamic organelles that fuse and divide in response to environmental stimuli, and particularly energy requirements. They receive instructions from nutrient sensing pathways, intracellular “weather stations” that pick up information from their environment, particularly on nutrient and hormone availability. The more nutrient-rich or hormone-rich the cellular environment is, the louder the messages they send to the mitochondria.
Communication overload
The default mitochondrial response to messages from sensing (also called signaling) pathways is to speed up in order to help clear out nutrients that are waiting to be churned into energy and that are taking up valuable intracellular space. Being constantly revved up, these energy motors start to malfunction, thereby failing to achieve their main function. Remember that mitochondria are a major source of oxidative stress, and that oxidation is a natural by-product of energy production. The higher their output in energy, the higher the amount of free radicals generated, which end up affecting vital mitochondrial structures like the matrix and the inner membrane, responsible for the oxidation of fatty acids and the electron transport chain, respectively.
When mitochondria are chronically revved up, they start to lose mass. Their own tissues begin to break down as a result of the oxidative burst they are exposed to, meaning that they end up failing to achieve their main function, i.e. energy production. ATP levels start to decrease as a result, and lack of cellular energy then leads to cellular ageing.
In order to resolve the communication overload that drives mitochondria into malfunction we need to go back to the source of the messages that tell them to work harder and faster and to churn out more energy. What we’re going to do is to “tone down” these messages so they’re no longer shouted out loud. Instead, we’re going to kindly request they become gentler, like whispers. In scientific terms, this is called modulation of the nutrient signaling pathways.
Dealing with excess
As we’ve seen in Figure 5 above, mitochondria cannot cope with excess very well. A perfect example of excess is consuming too many calories, possibly the best way to promote mitochondrial dysfunction and a regular part of most people’s lives in 2015. Caloric excess is particularly damaging when these calories are “empty”, i.e. devoid of nutrients. You know that not all calories are the same. For example, 1,000 calories from chocolate cake are not the same as 1,000 from a vegetable stir fry, because 1,000 calories from chocolate cake come mainly from sugar, and are devoid of any other nutrients and co-factors that have a protective effect on the mitochondria, like vitamins, minerals and antioxidant plant pigments in vegetables that protect mitochondrial structures from free radical damage.
Caloric excess is not the only type of excess that can lead to mitochondrial dysfunction. Signals from anabolic hormones (such as testosterone) and growth factors (such as growth hormone and insulin-like growth factors) are picked up by sensing/signaling pathways such as AMPK / mTOR (more on these in Part 2) and are translated into the same kind of commands for mitochondria to work faster churn out more energy. The intracellular environment has a very delicate equilibrium and can only take a certain level of nutrients and hormones at any given time. Mitochondria help clear these by “burning them” and creating energy as a result. This means that the more plentiful nutrients and hormones are inside cells, the more likely it is that mitochondria will go into a overdrive in an attempt to clear the excess and to turn it into energy, with the accompanying, unavoidable free radical damage that goes with it. A short spell of hyper function is OK for mitochondria to deal with. But when hyper function becomes chronic, malfunction, dysfunction, and loss of mitochondrial biomass follow suit, contributing to a loss of ATP that fuels cellular ageing and the development of diseases of ageing.
Stress + Hyperfunction + Ageing
As we’ve seen above, chronic activation of signaling pathways is triggered by over nutrition but also by hormonal excess. And we all know that psychosocial stress is a major hormonal driver. Cortisol, the “stress hormone”, is a protagonist in most people’s lives. Its role as a hormone is catabolic, i.e. it breaks down tissue. In fact, cortisol breaks down tissues like muscle into glucose, which feeds into the cycle we see in figures 6 and 7. This is important because the effects of stress on signaling pathways are comparable to those of over nutrition, as both trigger the same chain of events, as seen in Figure 7 below. Stress can still feed into the “hyper function cycle” by breaking tissue down, even if your diet is frugal. Cortisol also acts as a messenger that stimulates the release of anabolic hormones such as insulin and insulin-like growth factor 1, which also feed into this cycle.
Mitochondria are the only source of cellular energy (ATP) in the body, so their malfunctioning has a massive knock-on effect on cellular function. If cells haven’t got the energy they need to go about performing basic functions, such as communication, nutrition and detoxification, they’ll end up ageing prematurely and possibly dying prematurely too. So lack of ATP as a result of mitochondrial dysfunction has an effect that can end up being called a number of names: ageing, metabolic syndrome, neuro degeneration… Depending on your genes and our environment (including our diet), every one of us will experience mitochondrial dysfunction in a different way, although there are clear signs of it that apply across the board, as seen in the figure above.
These include, to name but a few:
- Fatigue, sometimes extreme, and often chronic.
- Lack of resilience, or ability to spring back after a stressful situation.
- Apathy, lack of libido, general disinterest in life, even depression.
- Accumulation of weight around the middle, even leading to increasing BMI that class as “obese”.
Hyper function no more… Key Nutrients.
Scientific research into the mechanisms driving cellular ageing agree that regulating mitochondrial function requires a multifaceted approach that includes a variety of bioactive compounds that are able to modulate nutrient signalling pathways by making their messages to mitochondria “less loud”, so these sensitive little organelles don’t feel pressurized to work overtime. One such bioactive is nicotinamide adenine dinucleotide, which exists in two forms. One is oxidized and is called NAD+ and the other one is reduced (i.e. less oxidizing) and is called NADH.
Think of mitochondrial dysfunction as a the result of mitochondria being sped up and pushed downhill by nutrient and hormonal excess and the forceful messages coming from nutrient sensing pathways, a bit like a car without breaks. NADH contributes to “putting a break on”, allowing for gentle yet effective mitochondrial function that doesn’t stretch the poor mitochondria’s boundaries beyond their limits by working over their safety threshold on a regular basis.
NADH and energy
NADH is an essential factor in the generation of ATP and in how energy is released from nutrients. For example, in order to generate energy from glucose and fatty acids these need to be oxidized. NAD+ is reduced to NADH as part of processes called beta oxidation and glycolysis, and also as part of the Krebs or citric acid cycle. NADH allows for electrons to be transported across the mitochondrial wall by the special mechanisms called shuttles, such as the malate-aspartate shuttle. NADH is then oxidized by the electron transport chain, which pumps protons across a membrane and generates ATP through oxidative phosphorylation. Perhaps these mechanisms make more sense now when you look back at Figure 5 above.
NADH and Sirtuins, the “fountain of youth” genes.
Aside from its role in energy production, NADH deserves to be considered a key nutrient in the preservation of “cellular youth” because of its role as a precursor of the sirtuin enzymes. Sirtuins (encoded by the genes SIRT1 to SIRT5) are tasked with the preservation of DNA integrity and stability and play an essential role in gene expression in all body tissues.
What is even more significant is that sirtuins also act as a break on signaling pathways such as mTOR. They slow down hyper functioning cells, preserving their health and saving them from a premature death.
CoQ10 the protector
CoQ10 is a vitamin-like, oil-soluble molecule, and its reduced form, known as ubiquinone, is a potent lipophilic antioxidant. Studies have demonstrated that CoQ10 has anti-oxidative effects and anti-ageing properties at the skin level and in spatial learning. CoQ10 is able to protect cells from ageing induced by high levels of intracellular free radicals generated by mitochondria. There’s also increasing evidence that CoQ10 can reverse mitochondrial dysfunction and decrease mitochondrial free radical generation.
What’s even more important is that emerging evidence shows that CoQ10 also works at the “root level” of the mTOR pathway by modulating its activity, i.e. following the same examples I used above, CoQ10 is able to “quieting down” the messages from signaling pathways to the mitochondria, which makes it a bit of a double-whammy nutrient that works both at the site of malfunction (the mitochondria) and at the root of the messages that lead to this malfunction.
NADH + CoQ10 together
Now we know about the benefits of NADH and CoQ10 on mitochondrial health and on signaling pathways, what would happen if we combined the two? A group of researchers from the Chronic Fatigue Syndrome Unit in the Vall D’Hebron Hospital in Barcelona had the same question. In order to get an answer, they conducted an 8-week, randomized, double-blind placebo-controlled trial to evaluate the benefits of oral CoQ10 (200 mg/day) plus NADH (20 mg/day) supplementation on fatigue and biochemical parameters in a group of 73 patients suffering from Chronic Fatigue and Fibromyalgia, two conditions that share mitochondrial dysfunction as mediating or perpetuating factors.
First of all, the biochemical analysis of blood samples from participants showed that oxidation of lipids in blood mononuclear cells, a marker for cellular ageing, had almost halved after 8 weeks, as seen in Figure 9 above. Additionally, ATP production also improved and, what’s even more interesting, citrate synthase, a marker of mitochondrial mass, was also dramatically enhanced.
Personally, the fact that this combination was able to increase mitochondrial biomass is the most significant finding. It literally means that mitochondria are able to rebuild tissue as a result, and this newly grown tissue allows them to regain integrity and function. I myself have started supplementing with a NADH and CoQ10 formula and have noticed an improvement in my energy levels and in my ability to deal with stressful situations. On the basis of my personal experience and the science behind it, I have started to use this nutrient combination in my clinics with those clients who warrant this type of intervention. I will be reporting back on my results! ReConnect
I’ll be exploring other angles to this story in Part 2, so stay tuned for that.
About Miguel
Miguel is a leading expert in Personalised Nutrition. His approach combines Systems Biology with Nutritional Therapy and Clinical Neuroscience. Miguel was the first UK Nutrition Professional to become Board Certified in Anti-Ageing, Functional and Regenerative Medicine (ABAAHP) and is the Chairman of the British Association for Applied Nutrition and Nutritional Therapy (BANT).
References
- Aging (Albany NY).2010 Aug;2(8):487-503.
- Adv Physiol Educ.2014 Jun;38(2):135-9.
- Aging (Albany NY).2010 May;2(5):265-73.
- J Clin Invest.2013 Mar;123(3):946-50.
- Oxid Med Cell Longev.2015;2015:867293.
- Antioxid Redox Signal.2015 Mar 10;22(8):679-85.






